S01.2: Relationships between nutrient storage and nutrient utilisation in long-term fasting birds and mammals
Yves Cherel1 & Rene Groscolas2
1Centre d'Etudes Biologiques de Chizé, UPR 4701 du Centre National de la Recherche Scientifique, F-79360 Villiers-en-Bois, France, fax +5 49 09 65 26, e-mail cherel@cebc.cnrs.fr; 2Centre d'Ecologie et Physiologie Energétiques, UPR 9010 du Centre National de la Recherche Scientifique, 23 rue Becquerel, F-67087 Strasbourg, France
Cherel, Y. & Groscolas, R. 1999. Relationships between nutrient storage and nutrient utilisation in long-term fasting birds and mammals. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:
17-34. Johannesburg: BirdLife South Africa.Birds and mammals adapt to prolonged fasting by mobilising fat stores and minimising protein loss. This strategy ends with an increase in protein utilisation associated with behavioural changes promoting food foraging. We review here the relationships between adiposity and the pattern of fuel utilisation during fasting. First, animals with small initial fat stores are unable to spare body proteins. Second, moderate fat stores initiate protein conservation. Third, higher initial adiposities enable longer fasts by allowing a more efficient and prolonged phase of protein sparing; however, there is a limit to the lowering of protein utilisation since proteins account for no less than 4% of energy expenditure. Lastly, accumulation of too much fat could be detrimental for survival to fasting, primarily because a lethal depletion of body proteins may be reached before foraging behaviour is stimulated by a critical depletion of fat stores. Birds are also able to adapt the level of fat and protein storage to the specific energy and nutrient needs of the subsequent periods of food deprivation. For example, King Penguin Aptenodytes patagonicus chicks build up more lipids before their long winter fast than do adults before the shorter incubating fast, and adult penguins build up more protein reserves before the molting fast during which they use endogenous protein for feather synthesis.
INTRODUCTION
The feasting and fasting way of life of many wild mammals and birds implies the storage and subsequent utilisation of endogenous nutrients, as indicated by their seasonal variations in body mass. Several groups of endotherms are well adapted for extremely long periods of food deprivation associated with reproduction, molt, migration, and hibernation (Mrosovsky & Sherry 1980; Castellini & Rea 1992). In birds, such proficient fasters are found within the order Sphenisciforms (penguins), Procellariiforms (albatrosses and petrels), and Anseriforms (ducks and geese), which regularly endure fasts of several weeks at the beginning of the breeding cycle, during courtship and incubation. The fact that some birds use previously stored energy for reproduction led to the concept of capital breeders in opposition to income breeders, i.e., organisms that use for reproduction energy acquired during the reproductive period itself (see review in Jönsson 1997). Birds, however, can also use nutrient stores outside the breeding period, thus extending the concept of capital breeders to capital molters, capital migrants, or capital winterers. Logically, fasting animals are good examples of capital organisms because they exclusively rely on endogenous nutrient stores to cover their energy expenditure, feeding and fasting being decoupled temporally, and often also spatially.
Observations and experiments on incubating birds and mammalian hibernators provided evidence that body mass is finely regulated during periods of hyperphagia and anorexia and that mass changes are programmed throughout the year, each body mass being appropriate to a particular phase of the cycle. Regulation occurs through variations in the programmed set-point for body fat, the main body component involved in mass changes. A reduction in set-point accounts for lowered intake and body mass loss occurring even in the presence of food (Mrosovsky & Sherry 1980).
Physiological investigations have pointed out the role of body fat in food-deprived animals. Fatter individuals fast longer and tolerate greater body mass loss than leaner individuals. The aim of the present work is to explain how interactions between fat stores and protein utilisation account for differences in fasting duration, metabolic adaptations, and behavioural response to prolonged fasting in fatty and lean organisms. Because adaptations to food deprivation are essentially the same in birds and mammals, including Humans (Homo sapiens), the review deals with results obtained on fasting normothermic endotherms with a special emphasis on birds, notably penguins. Fasting is here defined as a period during which animals do not feed at all, water remaining available ad libitum. We did not consider fasts characterized by large changes in energy expenditure, such as hibernation and torpor, which are marked by sharp drops in internal temperature, or migrations marked by a high locomotor activity. Instead, we focus on resting or moderately active animals within or close to their thermoneutral zone.
Three phases of fasting
From changes in the daily loss of body mass, the fast of penguins and Domestic Geese Anser anser has been divided into three phases (see review in Cherel et al. 1988): I, the daily body mass loss drops; II, it remains at a minimum level or decreases slightly; and III, it increases. These phases reflect different metabolic adjustments (Cherel et al. 1988; Groscolas 1990). Phase I is a rapid period of adaptation, marked by increasing mobilisation of fat stores and a lowering in protein utilisation. Phase II is a long phase of economy, during which most of the energy expenditure is derived from lipids while body proteins are efficiently spared. The remarkable long fast of some birds may be explained by their ability to prolong the metabolic state of phase II during several months (Cherel & Le Maho 1985; Robin et al. 1988b). At an advanced stage of fasting, however, this metabolic strategy ends with the occurrence of phase III, characterised by an increase in protein utilisation while fat stores are progressively exhausted. Note that time changes in daily protein utilisation parallel those in daily body mass loss during the three phases of fasting, which reflects the high water loss associated with protein degradation, but not lipolysis (Groscolas 1988).
Data in the literature show that the three phases of fasting can be used to describe how a wide variety of wild and domestic birds and mammals, including Laboratory Rats Rattus norvegicus and Humans, adapt to prolonged fasting (see review in Felig 1979; Goodman et al. 1980; Cherel et al. 1988; Castellini & Rea 1992). Metabolic characteristics of phase II correspond to the well-known adaptations of animals to withstand long-term food deprivation, i.e., a high level in lipid utilisation and a low level in protein loss. During phase II, fuel metabolism is in a steady-state as indicated by constant and linear decreases in lipid and protein masses over time; consequently, whatever the duration of phase II is, percentages of energy deriving from fat and protein utilisation do not vary during that period (Groscolas 1990; Groscolas et al. 1991; Cherel et al. 1995). On the other hand, both phases I and III are dynamic states. In the adaptive phase I, fuel metabolism reaches the steady-state that characterises phase II within several days, whereas in phase III animals burn progressively more and more endogenous proteins, and less and less triglycerides (Groscolas 1990).
Phase III of fasting is highly adaptive because changes in fuel utilisation are closely related to changes in behaviour anticipating a lethal depletion of energy stores and promoting food foraging (Groscolas 1986; Le Maho et al. 1988; Groscolas 1990). In captive Emperor Penguins Aptenodytes forsteri (Robin et al. 1998) and Laboratory Rats (Koubi et al. 1991), spontaneous locomotor activity greatly increases below the body mass of transition between phases II and III, the threshold critical body mass. This reflects a rise in the drive to refeed controlled by a 'refeeding signal,' probably linked to a metabolic and hormonal shift (Robin et al. 1998). From an ethical point of view, the total duration of phase III cannot be determined because it would require prolongation of the fast until a pathological and irreversible stage is reached (Cherel et al. 1988). However, an old investigation (Schimanski 1879) indicates that chickens Gallus gallus become motionless after about one week of increase in protein utilisation, while fasting geese, ducks, owls, and rats in one hand (Robin et al. 1988a, 1991; Cherel & Le Maho 1991; Handrich et al. 1993), and penguins on the other hand (Robin et al. 1998) tolerate a few days and 2-3 weeks in phase III, respectively, without injury. This indicates that, although phase III is reversible, it is critical since fasting birds and mammals must refeed or be refed before its end.
Recent findings on King Penguins Aptenodytes patagonicus (Olsson 1997) and Blue Petrels Halobaena caerulea (Chaurand & Weimerskirch 1994; Ancel et al. in press) have shown that the critical body mass is closely related to the body mass for which spontaneous desertion of the egg occurs in the field. Therefore, a causal link exists between the physiology of the birds and its behavioural ecology, the nutritional status of the fasting animal driving the trade-off between current reproduction and future survival. Such results emphasize, as stressed by McNamara and Houston (1996), the importance of the organism's physiological state on individual optimisation of life-history decisions.
Energy stores and duration of fasting
Birds, like mammals, can store energy in three biochemical forms: fat, protein, and carbohydrate (see review in Blem 1990). By far the more efficient form of storage and the largest reservoir of body fuel is in the form of fat, stored as triglycerides in depots of white adipose tissue. The caloric density of adipose tissue is high (36-38 kJ g-1) due to the high energy content of triglycerides (39-40 kJ g-1) and the low water content of the tissue. On the other hand, both carbohydrate and protein have a lower energy density, and their storage necessitates intracellular water accumulation, thus reducing the caloric content to 4-8 kJ g-1 of tissue (Cherel et al. 1988; Blem 1990). The main form of carbohydrate stores is glycogen located in liver and muscle. Glycogen is, however, an insignificant fuel storage in birds, representing less than 1% of total prefasting energy content in Emperor Penguins, whereas proteins account for 17-23%, and fat for the remainder (Groscolas 1982). Glycogen stores generally are useful only for short-term energy demands (Cherel et al. 1988; Blem 1990) and are, therefore, beyond the scope of this review.
In birds and mammals, the major reservoir of body proteins is the skeletal muscle tissue (Blem 1990), which acts as an important homeostatic organ by helping to maintain acceptable levels of amino acids and glucose in the circulation (Daniel et al. 1977). Unlike fat, however, protein generally is not viewed as an energy store because each molecule has a structural or functional role and is not, therefore, a biochemical form of storage per se (Felig 1979; Blem 1990). Consequently, the ideal situation for a fasting animal would be an ability to use only fat and totally spare body protein. However, although there is an important lowering of protein utilisation in phase I of fasting, some net endogenous protein degradation persists during phase II. Because fat utilisation and protein sparing are the major metabolic adaptations to long-term food deprivation, tolerance to fasting is mainly determined by duration of phase II. How long can animals spare their body protein at the expense of fat, and what factors control the duration of phase II are thus questions of primary importance to understanding how animals physiologically adapt to prolonged fasting.
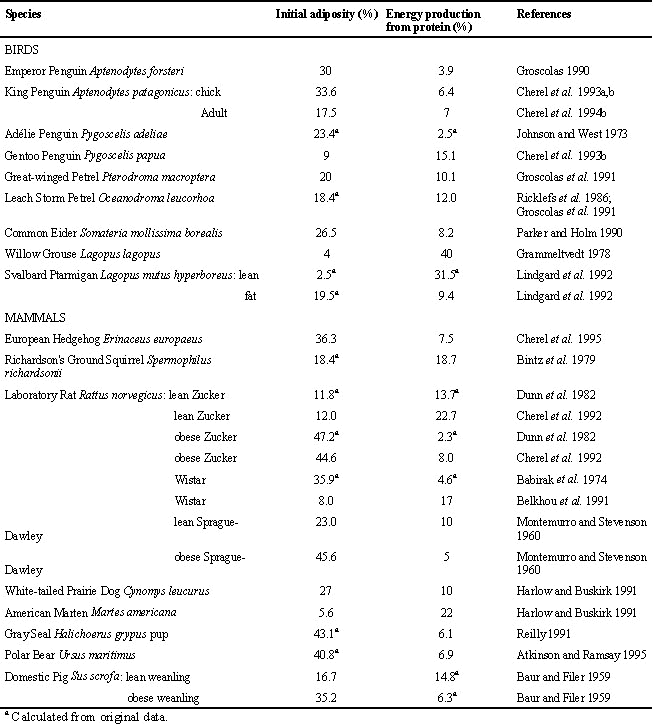
There is obviously a relationship between the duration of fasting and the amount of fat stores at the beginning of the fast: the higher the initial adiposity (mass of total lipids/body mass), the longer the fast. Within the same species, fatty individuals tolerate longer periods of food deprivation than their lean counterparts; this was found in birds (Boismenu et al. 1992; Lindgard et al. 1992) and mammals (Cuendet et al. 1975; Goodman et al 1980), including Humans (Barnard et al. 1969; Leiter & Marliss 1982). The relationship is also valid when comparing species of the same family. As shown in Table 1 and Fig. 1, fatty male Emperor Penguins tolerate longer natural fasts than leaner male Adélie Penguins Pygoscelis adéliae. The difference in the duration of fasting involves mainly the lengthening of phase II in fatter organisms (Cherel et al. 1992). Thus, a first interaction between lipid and protein during fasting is that a larger initial adiposity enables a longer fast through a longer phase of protein sparing.
A longer fast induces a higher total body mass loss and, consequently, fatty individuals tolerate greater body mass reduction than leaner individuals. For example, chicks and adults of King Penguins with an adiposity of 34% and 18%, respectively, can sustain 5 and 1.5 months of fasting corresponding to 68% and 41% decreases in mass (Cherel & Le Maho 1985; Cherel et al. 1993a, 1994b). The main explanation is that a large percentage of fat stores can be safely depleted; thus, obese animals tolerate a greater mass loss because they can use a larger amount of lipids over a longer time, and they also burn cumulatively larger amounts of body protein (see below).
Adiposity and effectiveness in protein sparingPrevious observations on fasting and semi-fasting Humans indicate that the relative contributions of lean body mass and fat to total body mass loss is a function of the body content of fat (Forbes 1987). Obese people lose proportionally less body nitrogen than thin people (Forbes & Drenick 1979) and, consequently, fat accounts for a higher percentage of energy expenditure than protein in obese Humans (Van Itallie & Yang 1977). Such inter-individual differences in the level of protein sparing in relation to lipid stores were also found in birds (Lindgard et al. 1992) and mammals (Cuendet et al. 1975; Goodman et al. 1980; Atkinson et al. 1996). A second interaction between lipid availability and protein utilisation is, therefore, that a larger amount of fat enables a higher efficiency in protein sparing during phase II.
Daily body mass loss and daily nitrogen excretion are constant or slightly decrease during phase II of fasting in the same individual, reflecting the metabolic steady-state occurring during the period of protein conservation (Robin et al. 1987, 1988b). The maintenance during phase II of a constant, low level of protein utilisation in the face of decreasing fat stores indicates that, at a given time of phase II, the level of protein loss is not governed by the current amount of fat; if it were, protein utilisation would progressively increase during phase II of fasting as fat stores decrease (Fig. 2). This means that the level of protein sparing is set up at the beginning of the fast, and is controlled by initial adiposity.
A simple way to compare protein sparing between individuals and species is to determine the proportion of the energy that is derived from protein during phase II (Cherel et al. 1995) or during the whole fasting period (mainly phase II) in species for which data are not available for phase II only. We reviewed the available literature on quantification of initial adiposity and subsequent fuel utilisation in fasting birds and mammals (Table 2), and we plotted relative energy production from protein according to initial body fat content for all the species (Fig. 3). The contribution of protein to energy expenditure ranges from about 4% in the fattest animals to 40% in the leanest, i.e., a 10-fold difference, and it decreases exponentially with increasing initial fatness. A similar relationship was recently found in semi-starved and prolonged starved Humans with an initial adiposity ranging from 5% to 55% (Dulloo 1997). Thus, better protein conservation occurs in fatter animals, not only within individuals of the same species, but also when comparing closely related species, different bird species, and even birds and mammals. Such a similarity in unrelated groups of fasting endotherms indicates a rather general physiological mechanism in which protein and lipid metabolism interact.
From data on very obese organisms, however, there is a limit in the effectiveness of protein conservation (about 4% of energy expenditure) that may initially be set up with accumulation of huge fat stores. Protein sparing is more and more effective when initial adiposity increases, especially for adiposity lower than about 20%, but an initial fatness of more than 35% does not appear to be a further advantage (Fig. 3). A physiological explanation of the minimum level of protein loss during fasting is the obligate requirement of certain tissues (mainly the brain) for some level of glucose consumption, which necessitates continuous formation of new glucose from endogenous precursors (amino acids from muscle protein and glycerol from triglycerides). A complete sparing of body proteins is thus never achieved, even in very obese Humans (Forbes & Drenick 1979) and animals (Cherel et al. 1992), including bears (Atkinson et al. 1996). In contrast to previous studies showing a total conservation of lean body mass in bears, it is now established that protein catabolism also meets a significant but small proportion of maintenance energy during winter dormancy (a period of reduced activity but near normal body temperature) (Atkinson et al. 1996). Bears nevertheless reach a very high level of protein sparing due to an almost complete reutilisation of urea produced (Barboza et al. 1997).
Adiposity, the three phases of fasting and physiological limits to starvationAdiposity controls not only fuel metabolism during phase II, but also the existence of the three phases of fasting. Animals with a low to very low initial adiposity are not able to conserve body protein during fasting, as indicated by no decrease or even an immediate increase of daily nitrogen excretion in response to food deprivation (Cuendet et al. 1975; Goodman et al. 1980; Lindgard et al. 1992). These animals tolerate only a few days of starvation, they show neither phase I nor phase II, and their metabolic response to food shortage is similar to a phase III because they immediately burn more and more protein over time (Lindgard et al. 1992).
In penguins and Laboratory Rats, fat stores are almost completely depleted when phase III is prolonged 2-3 weeks and a few days, respectively (Robin et al. 1988b; Belkhou et al. 1991; Cherel et al. 1992, 1994b). At the transition between phases II and III, however, there still remain substantial lipid stores in the body (18-25% of the initial fat content), indicating that the progressive increase in protein utilisation is not the mere consequence of lipid exhaustion. This, together with pharmacological studies, lead to the hypothesis that protein utilisation increases when a threshold adiposity is reached (see discussion in Cherel et al. 1992; Robin et al. 1998). This threshold adiposity could correspond to the state where adipose tissue, because of its reduced size, is no longer able to supply fatty acids at an output sufficient to meet almost entirely the energy needs of the body (Robin et al. 1998). In agreement with this concept, initial adiposity for which there is an immediate use of body proteins on fasting is lower (2-3%) (Lindgard et al. 1992) than threshold adiposity (3-9%) (Robin et al. 1988b; Belkhou et al. 1991; Cherel et al. 1992, 1994b). Whether regulatory mechanisms are identical at the beginning or toward the end of a fast in the control of increase in net protein catabolism, however, remains to be elucidated.
To further investigate the interactions between adiposity and protein utilisation, we compared fuel metabolism during prolonged fasting in lean and pathologically obese rats that had an initial fat content of 12% and 45%, respectively (Cherel et al. 1992). Fatty animals fasted longer (81 versus 15 days), tolerated a larger decrease in body mass (71% versus 44%), and better conserved body proteins (8% versus 23% of energy expenditure from protein). Importantly, they did not have a late increase in protein loss (Cherel et al. 1992) and, accordingly, had no behavioural changes that are associated with phase III (Robin, Cherel & Le Maho unpublished results). Accumulation of too much fat is therefore detrimental for survival because it eliminates the late phase of increase in nitrogen excretion that is linked to a food foraging behaviour anticipating a lethal depletion of energy stores. Body composition analysis explains the lack of phase III in fatty animals. At the end of the experiment, they still had a body fat content (27%) far above the threshold adiposity inducing the late increase in protein utilisation (Cherel et al. 1992).
Our study on fatty animals also explains the apparent conflicting data in the literature regarding the metabolic limitations of prolonged fasting, i.e., either protein loss (Felig 1979) or lipid depletion (Leiter and Marliss 1982). In very fat animals, a lethal cumulative loss of body protein restricts the length of starvation, while fat stores are still available. On the other hand, survival in leaner organisms depends primarily on the availability of lipids because fatty acid availability is essential to supply the energy required for gluconeogenesis (see discussion in Cherel et al. 1992). Accordingly, birds have died from starvation in the wild when they exhausted their fat stores, but not necessarily their protein reserves (Davidson and Clark 1985). Body composition analysis at that time indicates that birds are cachectic and very lean (adiposity < 1.5%) (Marcström & Mascher 1979; Piersma 1984; Jenni-Eiermann & Schifferli 1989), most of the remaining lipids being probably not adipose triglycerides but structural compounds (Cherel et al. 1992).
Interactions between adiposity and fuel metabolism during fasting: an integrative viewThe similarity of the interactions between initial adiposity and fuel metabolism during prolonged fasting in various species of birds and mammals indicates a rather general physiological picture of adapting efficiently to long-term food deprivation. The effects of increasing initial amounts of fat on these interactions may be summarised as follows (Fig. 4):
- a very low initial fatness (a), see Fig. 4, less than or equal to the threshold adiposity of about 5-10%, induces an immediate and rapid use of endogenous body proteins. There are no phases I and II, and animals only tolerate a few days of starvation and a small decrease in body mass;
- an initial fat content above the threshold adiposity (b) allows the sparing of body protein. Animals show the typical pattern of three successive phases of fasting.
- if initial adiposity further increases (c), protein conservation is more effective, and animals tolerate a higher total body mass loss and a longer fast through a longer phase II. When threshold adiposity is reached, animals burn more and more protein (phase III), and a 'signal for refeeding' promotes a food foraging behaviour instead of continuing the activity associated with the fast (such as incubation).
- a still higher initial adiposity (d), roughly above 30-35%, induces only a lengthening of fasting without a further lowering of protein utilisation in phase II. Effectiveness in protein sparing is set up at the incompressible minimum level of protein loss that remains independent of larger initial lipid stores. The threshold adiposity is still reached before a lethal depletion of body protein occurs, thus inducing metabolic and behavioural changes associated with phase III.
- a very high initial adiposity (e) leads to an extended fast and a very important total body mass and protein losses, although protein sparing is maximum. Importantly, the threshold adiposity is not reached before a lethal depletion of body protein occurs, and animals die of starvation while substantial fat stores are still available. There is no phase III, and thus no 'signal for refeeding' associated with the late increase in protein loss and, consequently, no behavioural changes promoting foraging.
In the wild, adiposity ranges widely, depending on the species and, for a given species, on the period of the life cycle (Blem 1990); however, fat content rarely exceeds 45-50% of fresh body mass (see, nevertheless, Piersma & Gill 1998). Such elevated adiposities are found in obese people and laboratory and domestic animals (Robin 1989). Cumulative storage of fat over very long periods without periodic set up to a leaner body composition leads to severe obesity that is quite different from the storage/utilisation cycle of endogenous nutrients in wild animals. Accumulation of too much fat is not only detrimental during fasting because it eliminates phase III and the associated 'signal for refeeding,' but also because it probably increases the physiological and ecological costs associated with lipid stores. A detailed account of the various costs of avian fat storage is beyond the scope of this review, but they include an increase in mass-dependent energy expenditure, predation risk, and foraging (Witter & Cuthill 1993). Thus, there is a trade-off between storing large amounts of energy to increase the duration of the fast, and limiting fat stores to reduce the cost of storage and to allow the 'refeeding signal' to be triggered. Such a trade-off seems precisely achieved in the Emperor Penguin during its breeding fast. Indeed, the body mass at which the female and the male usually end their fasts and go to sea to refeed, after laying and after being relieved by its mate, respectively, is the same as the critical body mass at the phase II - phase III transition (Groscolas 1990).
Differential nutrient storage and utilisationEnergy stores used during fasting depend not only on the level of energy expenditure and the length of the period of food deprivation, but also on specific nutrient needs related to activities associated with the fast, such as reproduction and molt. An interesting point, therefore, is to know if animals store energy nonadaptively by building up nutrient stores in excess or if they adapt the level of energy stored to the specific needs of the subsequent fast. Because there are mass-dependent costs associated with energy storage (Witter & Cuthill 1993), theoretically the most beneficial strategy should be to adapt the level of nutrient storage as close as possible to, or slightly above, the level of nutrient needs.
To test this hypothesis, we compared body stores buildup in King Penguins before three distinct periods of food deprivation: the long winter fast of the chicks, and the breeding and molting fasts of the adults (Cherel et al. 1993a; Cherel 1995). Chick winter fast and adult breeding fast are periods of food deprivation during which there are no special nutrient requirements, the main difference between them being that chicks tolerate a longer period of food deprivation and a higher body mass loss than adults (Cherel et al. 1987, 1994b). On the other hand, adult penguin molt is quite unusual among birds because they renew their entire plumage during a complete and prolonged fast. Consequently, penguins must rely on endogenous fat and protein to support not only fasting energy expenditure but also the energetic and protein costs of feather synthesis (Groscolas & Cherel 1992; Cherel et al. 1994a).
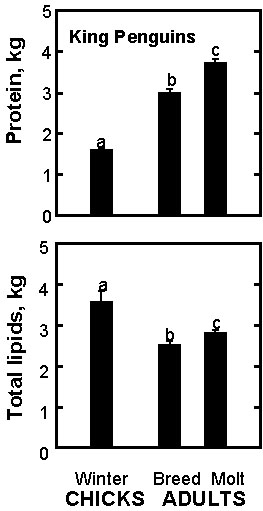
Body composition analysis indicates that, despite a lower prefasting body mass (10.6 versus 14.6 kg), chicks build up larger fat stores (3.6 versus 2.5 kg) and, consequently, have a much higher prefasting adiposity (34% versus 18%) than prebreeding adults (Fig. 5). Due to the interactions between fat and protein metabolism depicted above, a higher initial fat content explains the tolerance of chicks to a longer fast. During spring, chicks invest preferentially food energy to store fat at the expense of pectoral-muscle growth, thus anticipating the long winter fast (Cherel et al. 1993a).
When compared to prebreeding birds, premolting penguins are heavier (17.8 versus 14.6 kg) and accordingly have higher total lipid (2.8 versus 2.5 kg) and protein (3.7 versus 3.0 kg) contents (Fig. 5). Most of the mass increase (90%), however, involves protein (23%) and its associated water (67%), fat being only a minor component of the increase (9%). Additional protein reserves, mainly located in pectoral muscles, thus anticipate the use of endogenous body protein for new feather synthesis during the molting fast (Cherel et al. 1993a).
A close comparison of energy stored before and utilised during breeding and molting fasts of King Penguins further indicates that more energy is stored at the beginning of the breeding cycle than utilised during the fast, but that the reverse is true for the molting period. This suggests not only short-term but also long-term regulation in nutrient stores (Cherel 1995). The breeding fast usually ends with relief by the mate when mean body mass is above critical body mass (Olsson 1997), leaving a safety margin in energy stores for incubation if the mate delays its return. On the other hand, molt is a more stressful metabolic situation for King Penguins. First, not only body fat but also protein are massively depleted, as shown by the mean body mass at the end of molt (Weimerskirch et al. 1992) often being below the critical body mass measured in breeding birds, and second, a transient decrease in thermal insulation precludes going to refeed at sea before the end of the molting process.
To our knowledge, this study is one of the first to show that, within the same avian species, different amounts of fat and protein are adaptively built up in anticipation of complete fasts of different durations and nutrient needs. In the same way, a wader, the Bar-tailed Godwit (Limosa lapponica), anticipate migrating flights of different distances by storing different amounts of fat before taking off: the longer the flight, the higher the initial adiposity (Piersma in press). Adaptive flexibility in nutrient storage awaits more data and similar types of analyses for other fasting wild species involved in activities with different energy and nutrient needs. The present work does, however, complement previous research showing that, within a species, fat stores generally increase with the unpredictability of food and that individuals become fatter during winter months compared to summer, therefore supporting the view that birds actively regulate their body mass and anticipate their energetic requirements (Mrosovsky & Sherry 1980; Witter & Cuthill 1993). Lastly, the fact that we observed in King Penguins a cyclic and predictable accumulation/utilisation of body protein together with fat strongly supports the concept of a labile protein reserve, mainly located in pectoral muscles in birds. This and the incompressible protein loss in phase II argues against the common view that protein is not readily mobilised and is used only after the nearly or complete utilisation of fat stores (Blem 1990). Penguins and other animals are, however, also able to mobilise body protein further during phase III, when fat stores are nearly exhausted.
CONCLUSION AND PERSPECTIVESThis review highlights the relationships between fat and protein metabolism during prolonged fasting and attempts to generalise these interactions to endothermic animals. Although birds and mammals adapt to food deprivation through identical metabolic changes, some additional physiological data are needed on birds to complete the picture. Due to the high adaptive value of phase III, for example, its complete absence in fatty Laboratory Rats (Cherel et al. 1992) must be verified on obese birds. Another potentially fruitful way would be to investigate the 'signal for refeeding' and its relationships with metabolic, endocrine, and behavioural changes occurring in late fasting. This indeed offers an exceptional opportunity to understand the long-term interregulation of body mass and food intake in endotherms.
Long-distance migrants were not considered here, although their moderately long fasts associated with a high energy expenditure due to flight costs show similarities with long-term 'inactive' fasts. Migrating birds build up and subsequently utilise large fat stores, some of them having an initial adiposity close to 50% (Piersma & Gill 1998). Based on data on fasting 'inactive' organisms, such huge fat stores seem incompatible with the occurrence of phase III of fasting and its associated 'signal for refeeding' described in less obese birds during 'inactive' fasts. Two hypothesis can explain this apparent discrepancy. First, migrants better spare their endogenous protein than 'inactive' birds. The few available data does not agree with this, since migrating birds that fast seem to adapt to food deprivation by attaining contribution of lipids to energy expenditure of about 95%, the proportional contribution of protein to energy expenditure decreasing with increasing initial adiposity, as in 'inactive' fasters (see review in Jenni & Jenni-Eiermann in press). Second, in the premigratory period, birds store fat but they also build up additional protein reserves which could help preventing the sudden occurrence of a lethal depletion of endogenous proteins. Indeed, compared to leaner birds, very obese migrants have the largest relative sizes of breast muscles with respect to fat-free mass (Piersma & Gill 1998). In view of the paucity of the existing data (Jenni & Jenni-Eiermann in press), more information is clearly needed on the quantification of fuel storage and fuel utilisation in long-distance migrants in the field, together with control experiments to determine body composition at the so-called critical body mass (transition phase II - phase III) for these species.
The existence of a lower threshold in body condition (the critical body mass), associated with egg desertion in penguins and petrels, is a concept of broad interest linking physiology and the trade-offs of life-history theory (in that case, between current reproduction and adult survival). Other activities are thus expected to be controlled by this lower threshold in nutrient stores. For example, it has been hypothetised that migratory movements of waders in the face of cold spells are regulated by endogenous nutrient depletion identical to that previously described in penguins (Piersma & Poot 1993). The level of energy storage, and not only the level of energy depletion, can also be involved in the control of reproduction. In capital breeders, only individuals attaining an upper threshold in body condition may possibly decide to breed, as suggested for Anseriform (Drent & Daan 1980) and Procellariiform (Weimerskirch 1992; Chastel et al. 1995) birds. To our knowledge, however, such an upper threshold has been recently demonstrated in only one species of vertebrates, a snake (Naulleau & Bonnet 1996). Clearly, more field-conducted and experimental studies are required to describe and understand the ecological consequences of the relationships between fuel storage, fuel utilisation, and behaviour in birds.
ACKNOWLEDGEMENTS The authors especially wish to thank J.P. Robin, Y. Le Maho and Y. Handrich for valuable discussions and arguments during the development of studies and experiments over the last 15 years. Field work on Antarctic and Subantarctic birds was supported financially and logistically by the Institut Français pour la Recherche et la Technologie Polaires and the Terres Australes et Antarctiques Françaises.REFERENCES
Ancel, A., Petter, L. & Groscolas, R. In press. Changes in egg and body temperature indicate triggering of egg desertion at a body mass threshold in fasting incubating Blue Petrels (Halobaena caerulea). Journal of Comparative Physiology B.
Atkinson, S.N. & Ramsay, M.A. 1995. The effects of prolonged fasting on the body composition and reproductive success of female Polar Bears (Ursus maritimus). Functional Ecology 9: 559-567.
Atkinson, S.N., Nelson, R.A. & Ramsay, M.A. 1996. Changes in the body composition of fasting Polar Bears (Ursus maritimus): the effect of relative fatness on protein conservation. Physiological Zoology 69: 304-316.
Babirak, S.P., Dowell, R.T. & Oscal, L.B. 1974. Total fasting and total fasting plus exercise: effects on body composition of the Rat. Journal of Nutrition 104: 452-457.
Barboza, P.S., Farley, S.D. & Robbins, C.T. 1997. Whole-body urea cycling and protein turnover during hyperphagia and dormancy in growing bears (Ursus americanus and U. arctos). Canadian Journal of Zoology 75: 2129-2136.
Barnard, D.L., Ford, J., Garnett, E.S., Mardell, R.J. & Whyman, A.E. 1969. Changes in body composition produced by prolonged total starvation and refeeding. Metabolism 18: 564-569.
Baur, L.S. & Filer L.J. 1959. Influence of body composition of weanling Pigs on survival under stress. Journal of Nutrition 69: 128-134.
Belkhou, R., Cherel, Y., Heitz, A., Robin, J.P. & Le Maho, Y. 1991. Energy contribution of proteins and lipids during prolonged fasting in the Rat. Nutrition Research 11: 365-374.
Bintz, G.L., Palmer, D.L., Mackin, W.W. & Blanton, F.Y. 1979. Selective tissue catabolism and water balance during starvation in Richardson's Ground Squirrels. Comparative Biochemistry and Physiology A 64: 399-403.
Blem, C.R. 1990. Avian energy storage. In: Power, D.M. (ed) Current Ornithology, Vol 7; New York;Plenum:59-113.
Boismenu, C., Gauthier, G. & Larochelle, J. 1992. Physiology of prolonged fasting in Greater Snow Geese (Chen caerulescens atlantica). The Auk 109: 511-521.
Castellini, M.A. & Rea, L.D. 1992. The biochemistry of natural fasting at its limits. Experientia 48: 575-582.
Chastel, O., Weimerskirch, H. & Jouventin, P. 1995. Influence of body condition on reproductive decision and reproductive success in the Blue Petrel. The Auk 112: 964-972.
Chaurand, T. & Weimerskirch, H. 1994. Incubation routine, body mass regulation and egg neglect in the Blue Petrel Halobaena caerulea. Ibis 136: 285-290.
Cherel, Y. 1995. Nutrient reserve storage, energetics, and food consumption during the prebreeding and premoulting foraging periods of King Penguins. Polar Biology 15: 209-214.
Cherel, Y., Charrassin, J.B. & Challet, E. 1994a. Energy and protein requirements for molt in the King Penguin Aptenodytes patagonicus. American Journal of Physiology 266: R1182-R1188.
Cherel, Y., Charrassin, J.B. & Handrich, Y. 1993a. Comparison of body reserve buildup in prefasting chicks and adults of King Penguins (Aptenodytes patagonicus). Physiological Zoology 66: 750-770.
Cherel, Y., El Omari, B., Le Maho, Y. & Saboureau, M. 1995. Protein and lipid utilisation during fasting with shallow and deep hypothermia in the European Hedgehog (Erinaceus europeus). Journal of Comparative Physiology B 164: 653-658.
Cherel, Y., Fréby, F., Gilles, J. & Robin, J.P. 1993b. Comparative fuel metabolism in Gentoo and King Penguins: adaptation to brief versus prolonged fasting. Polar Biology 13: 263-269.
Cherel, Y., Gilles, J., Handrich, Y. & Le Maho, Y. 1994b. Nutrient reserve dynamics and energetics during long-term fasting in the King Penguin (Aptenodytes patagonicus). Journal of Zoology (London) 234: 1-12.
Cherel, Y. & Le Maho, Y. 1985. Five months of fasting in King Penguin chicks: body mass loss and fuel metabolism. American Journal of Physiology 249: R387-R392.
Cherel, Y. & Le Maho, Y. 1991. Refeeding after the late increase in nitrogen excretion during prolonged fasting in the Rat. Physiology and Behavior 50: 345-349.
Cherel, Y., Robin, J.P., Heitz, A., Calgari, C. & Le Maho, Y. 1992. Relationships between lipid availability and protein utilisation during prolonged fasting. Journal of Comparative Physiology B 162: 305-313.
Cherel, Y., Robin, J.P. & Le Maho, Y. 1988. Physiology and biochemistry of long-term fasting in birds. Canadian Journal of Zoology 66: 159-166.
Cherel, Y., Stahl, J.C. & Le Maho, Y. 1987. Ecology and physiology of fasting in King Penguin chicks. Auk 104: 254-262.
Cuendet, G.S., Loten, E.G., Cameron, D.P., Renold, A.E. & Marliss, E.B. 1975. Hormone-substrate response to total fasting in lean and obese mice. American Journal of Physiology 228: 276-283.
Daniel, P.M., Pratt, O.E. & Spargo, E. 1977. The metabolic homoeostatic role of muscle and its function as a store of protein. The Lancet 2: 446-448.
Davidson, N.C. & Clark, N.A. 1985. The effects of severe weather in January and February 1985 on waders in Britain. Wader Study Group Bulletin 44: 10-16.
Drent, R.H. & Daan, S. 1980. The prudent parent: energetic adjustments in avian breeding. Ardea 68: 225-252.
Dulloo, A.G. 1997. Human pattern of food intake and fuel-partitioning during weight recovery after starvation: a theory of autoregulation of body composition. Proceedings of the Nutrition Society 56: 25-40.
Dunn, M.A., Houtz, S.K. & Hartsook, E.W. 1982. Effects of fasting on muscle protein turnover, the composition of weight loss, and energy balance of obese and nonobese Zucker Rats. Journal of Nutrition 112: 1862-1875.
Felig, P. 1979. Starvation. In: De Groot, L.J. (ed) Endocrinology; vol. 3. New York; Grune and Stratton: 1927-1940.
Forbes, G.B. 1987. Lean body mass-body fat interrelationships in Humans. Nutrition Reviews 45: 225-231.
Forbes, G.B. & Drenick, E.J. 1979. Loss of body nitrogen on fasting. The American Journal of Clinical Nutrition 32: 1570-1574.
Goodman, M.N., Larsen, P.R., Kaplan, M.M., Aoki, T.T., Young, V.R. & Ruderman, N.B. 1980. Starvation in the rat. II. Effect of age and obesity on protein sparing and fuel metabolism. American Journal of Physiology 239: E277-E286.
Grammeltvedt, R. 1978. Atrophy of a breast muscle with a single fibre type (M. pectoralis) in fasting Willow Grouse, Lagopus lagopus (L.). Journal of Experimental Zoology 205: 195-204.
Groscolas, R. 1982. Modifications métaboliques et hormonales en relation avec le jeûne prolongé, la reproduction et la mue chez le Manchot empereur (Aptenodytes forsteri). Thèse d'Etat, Université de Dijon, France.
Groscolas, R. 1986. Changes in body mass, body temperature and plasma fuel levels during the natural breeding fast in male and female Emperor Penguins. Journal of Comparative Physiology B 156: 521-527.
Groscolas, R. 1988. The use of body mass loss to estimate metabolic rate in fasting sea birds: a critical examination based on Emperor Penguins (Aptenodytes forsteri). Comparative Biochemistry and Physiology 90A: 361-366.
Groscolas, R. 1990. Metabolic adaptations to fasting in Emperor and King Penguins. In: Davis, L.S., Darby J.T. (eds) Penguin Biology. San Diego; Academic Press: 269-296.
Groscolas, R. & Cherel, Y. 1992. How to molt while fasting in the cold: the metabolic and hormonal adaptations of Emperor and King penguins. Ornis Scandinavica 23: 328-334.
Groscolas, R., Schreiber, L. & Morin, F. 1991. The use of tritiated water to determine protein and lipid utilisation in fasting birds: a validation study in incubating Great-winged Petrels, Pterodroma macroptera. Physiological Zoology 64: 1217-1233.
Handrich, Y., Nicolas, L. & Le Maho, Y. 1993. Winter starvation in captive common Barn-Owls: physiological states and reversible limits. The Auk 110: 458-469.
Harlow, H.J. & Buskirk, S.W. 1991. Comparative plasma and urine chemistry of fasting White-tailed Prairie Dogs (Cynomys leucurus) and American Martens (Martes americana): representative fat- and lean-bodied animals. Physiological Zoology 64: 1262-1278.
Isenmann, P. 1971. Contribution à l'éthologie et à l'écologie du Manchot empereur (Aptenodytes forsteri Gray) à la colonie de Pointe Géologie (Terre Adélie). L'Oiseau et la Revue Française d'Ornithologie 41: 9-64.
Jenni, L. & Jenni-Eiermann, S. In press. What to take on board: metabolic constraints and adaptations of fuel supply in migrating birds. Journal of Avian Biology.
Jenni-Eiermann, S. & Schifferli, L. 1989. Body composition of starved Tufted Ducks Aythia fuligula, Pochards A. ferina, and Little Grebes Tachybaptus ruficollis. Wildfowl 40: 99-105.
Johnson, S.R. & West, G.C. 1973. Fat content, fatty acid composition and estimates of energy metabolism of Adélie Penguins (Pygoscelis adeliae) during the early breeding season fast. Comparative Biochemistry and Physiology B 45: 709-719.
Jönsson, I. 1997. Capital and income breeding as alternative tactics of resource use in reproduction. Oïkos 78: 57-66.
Koubi, H.E., Robin, J.P., Dewasmes, G., Le Maho, Y., Frutoso, J. & Minaire, Y. 1991. Fasting-induced rise in locomotor activity in Rats coincides with increased protein utilisation. Physiology and Behavior 50: 337-343.
Leiter, L.A. & Marliss, E.B. 1982. Survival during fasting may depend on fat as well as protein stores. Journal of American Medical Association 248: 2306-2307.
Le Maho, Y., Robin, J.P. & Cherel, Y. 1988. Starvation as a treatment for obesity: the need to conserve body protein. News in Physiological Sciences 3:21-24.
Lindgard, K., Stokkan, K.A., Le Maho, Y. & Groscolas, R. 1992. Protein utilisation during starvation in fat and lean Svalbard Ptarmigan (Lagopus mutus hyperboreus). Journal of Comparative Physiology B 162: 607-613.
McNamara, J.M. & Houston, A.I. 1996. State-dependent life histories. Nature 380: 215-221.
Marcström, V. & Mascher, J.W. 1979. Weights and fat in Lapwings Vanellus vanellus and Oystercatchers Haematopus ostralegus starved to death during a cold spell in spring. Ornis Scandinavica 10: 235-240.
Montemurro, D.G. & Stevenson, J.A. 1960. Survival and body composition of normal and hypothalamic obese Rats in acute starvation. American Journal of Physiology 198: 757-761.
Mrosovsky, N. & Sherry, D.F. 1980. Animal anorexias. Science 207: 837-842.
Naulleau, G. & Bonnet, X. 1996. Body condition index for breeding in a viviparous snake. Oecologia 107: 301-306.
Olsson, O. 1997. Clutch abandonment: a state-dependent decision in King Penguins. Journal of Avian Biology 28: 264-267.
Parker, H. & Holm, H. 1990. Patterns of nutrient and energy expenditure in female Common Eiders nesting in the high arctic. The Auk 107: 660-668.
Piersma, T. 1984. Estimating energy reserves of Great Crested Grebes Podiceps cristatus on the basis of body dimensions. Ardea 72: 119-126.
Piersma, T. In press. Phenotypic flexibility during migration: optimization of organ size contingent on the risks and rewards of fueling and flight? Journal of Avian Biology.
Piersma, T. & Gill, R.E. 1998. Guts don't fly: small digestive organs in obese Bar-tailed Godwits. The Auk 115: 196-203.
Piersma, T. & Poot, M. 1993. Where waders may parallel penguins: spontaneous increase in locomotor activity triggered by fat depletion in a voluntarily fasting Knot. Ardea 81: 1-8.
Reilly, J.J. 1991. Adaptations to prolonged fasting in free-living weaned seal pups. American Journal of Physiology 260: R267-R272.
Ricklefs, R.E., Roby, D.D. & Williams, J.B. 1986. Daily energy expenditure by adult Leach's Storm-petrels during the nesting cycle. Physiological Zoology 59: 649-660.
Robin, J.P. 1989. Modifications métaboliques et comportementales au cours du jeûne prolongé. Réalimentation après un jeûne prolongé. Thèse d'Université, Université Louis Pasteur de Strasbourg.
Robin, J.P., Boucontet, L., Chillet, P. & Groscolas, R. 1998. Behavioral changes in fasting Emperor Penguins: evidence for a 'refeeding signal' linked to a metabolic shift. American Journal of Physiology 274: R746-R753.
Robin, J.P., Cherel, Y., Girard, H., Chaban, C. & Le Maho, Y. 1988a. Augmentation du rendement azoté et hyperphagie associées à la réalimentation après un jeûne prolongé chez l'Oie domestique. Comptes Rendus de l'Académie des Sciences de Paris, Série III, 306: 375-379.
Robin, J.P., Cherel, Y., Girard, H., Geloen, A. & Le Maho, Y. 1987. Uric acid and urea in relation to protein catabolism in long-term fasting geese. Journal of Comparative Physiology B 157: 491-499.
Robin, J.P., Frain, M., Sardet, C., Groscolas, R. & Le Maho, Y. 1988b. Protein and lipid utilisation during long-term fasting in Emperor Penguins. American Journal of Physiology 254: R61-R68.
Robin, J.P., Zorn, T. & Le Maho, Y. 1991. Résistance au jeûne hivernal et réalimentation chez le Canard colvert: évolution des réserves énergétiques et de la prise alimentaire. Comptes Rendus de l'Académie des Sciences de Paris, Série III, 313: 529-535.
Schimanski, H. 1879. Der Inanitions- und Fieberstoffwechsel der Hühner. Zeitschrift für physiol. Chemie 3: 396-421.
Stonehouse, B. 1960. The King Penguin Aptenodytes patagonica of South Georgia. I. Breeding behaviour and development. Falkland Islands Dependencies Survey, Scientific Reports 23: 1-81.
Ulbricht, J. & Zippel, D. 1994. Delayed laying and prolonged fasting in Adélie Penguins Pygoscelis adeliae. Polar Biology 14: 215-217.
Van Itallie, T.B. & Yang, M.U. 1977. Diet and weight loss. The New England Journal of Medicine 297: 1158-1161.
Weimerskirch, H. 1992. Reproductive effort in long-lived birds: age-specific patterns of condition, reproduction and survival in the Wandering Albatross. Oïkos 64: 464-473.
Weimerskirch, H., Stahl, J.C. & Jouventin, P. 1992. The breeding biology and population dynamics of King Penguins Aptenodytes patagonica on the Crozet Islands. The Ibis 134: 107-117.
Witter, M.S. & Cuthill, I.C. 1993. The ecological costs of avian fat storage. Philosophical Transactions of the Royal Society of London, Series B 340: 73-92.
Table 1. Initial adiposity and protein utilisation (% of total energy expenditure) during either phase ii of fasting or the whole fasting period in birds and mammals.

Table 2. Initial adiposity and total duration of fasting of penguins in their breeding colonies
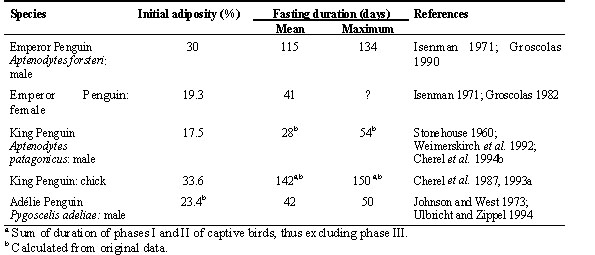
Fig. 1. Relationship between initial adiposity and duration of natural fasting in penguins (KPC: King Penguin chicks; KPM: King Penguin males; EPM: Emperor Penguin males; EPF: Emperor Penguin females; APM: Adélie Penguin males).
Fig. 2. Observed effect of initial adiposity (continuous line) and theoretical effect of current adiposity (dashed line) on protein utilisation during phase II of fasting. Theoretical line represents changes in protein utilisation if determined by current adiposity in phase II, i.e. an increasing protein utilisation with decreasing fat stores, as observed during phase III.
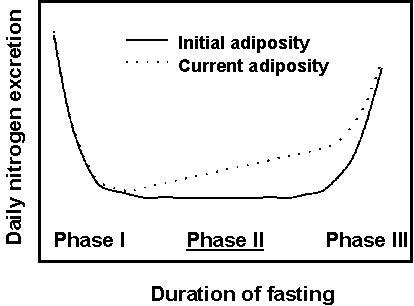
Fig. 3. Protein utilisation during phase II of fasting or the whole fasting period plotted against initial body fat content for various species of birds and mammals. Protein utilisation is expressed as its relative contribution to energy expenditure, and adiposity was calculated as the percentage of total lipid mass/body mass.
Fig. 4. A summary of the effects of increasing initial adiposity on protein utilisation (expressed as daily nitrogen excretion) during prolonged fasting (see text for adiposities a, b, c, d, and e).
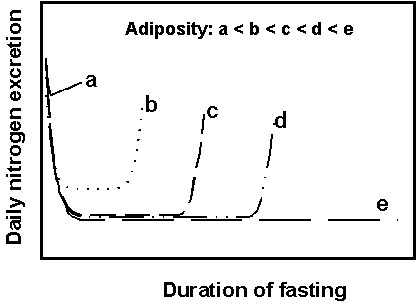
Fig. 5. Prefasting energy stores in winter chicks and in breeding and molting adults of King Penguins. Values not sharing a common superscript letter are significantly different (P < 0.05).