S01.1: Evaluation of some nonlethal methods of estimating avian body fat and lean mass
James A. Gessaman
Department of Biology and Ecology Center, Utah State University, Logan, Utah, USA 84322-5305, fax 435-797-1575, e-mail fajimg@cc.usu.edu
Gessaman, J.A. 1999. Evaluation of some nonlethal methods of estimating avian body fat and lean mass. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 2-16. Johannesburg: BirdLife South Africa.
The accuracy and limitations of five nonlethal methods of estimating either lean mass (LM) or fat mass (FM) of animals are evaluated for birds. The isotopic water dilution method of estimating LM and protein content of a live bird from total body water (TBW) and %FM from %TBW, although used in only a few avian studies, deserves a more critical evaluation of its accuracy on a broad range of avian species. Whole-body potassium-40 counting, which is used to estimate the body composition of humans with only a 1 to 2% error, is not applicable for birds; their whole body potassium-40 is too low. The cyclopropane absorption method, which can be calibrated with olive oil rather than bird fat, estimates the FM of Rock Doves with a mean error of 11%. The disadvantages of this method for field studies outweigh the advantages. The error of predicting LM and FM with the total body electrical conductivity (TOBEC) method alone is 1 to 9% and 10 to >300%, respectively. For some species, the TOBEC index explains 2 to 8% of the variance in FM not accounted for by body mass or body size parameters in multiple regression equations. The multiple regression approach of estimating LM or FM, using combinations of independent variables, such as body mass, body size parameters, TOBEC indices, and body water, is currently the most accurate nonlethal method available for field studies of avian body composition.
INTRODUCTION
A complete understanding of the ecological energetics of an avian species requires measurements of the energy flux dynamics (energy mobilization and storage) of body fat and protein in individual birds over time. The total energy and nutrient content of a bird’s body can be partitioned into two compartments: body stores and body structural components. Stores are the nutrients (viz., fat and protein) and the energy accumulated in anticipation of periods of shortage. Fat is the main energy store in birds (Pond 1981; Blem 1990), with an energy content about 2.5 times that of protein (stored or structural) or carbohydrate (glycogen) per unit dry mass and about 8 times per unit wet mass. Generally, the majority of the mobilisable energy in birds is stored as fat; however, this may not be the case during phase III of extended fasting (Cherel & Groscolas 1999). The most accurate method of measuring the fat mass (FM) of a bird, which is nearly all stored fat, and the lean mass (LM), which contains more structural than stored protein, requires sacrificing the animal and extracting its body fat with a solvent such as petroleum ether; however, killing any subject animal is undesirable. This problem has prompted researchers to evaluate several nonlethal (and typically noninvasive) methods of estimating body fat and lean mass of birds (see Brown 1996 for a review).
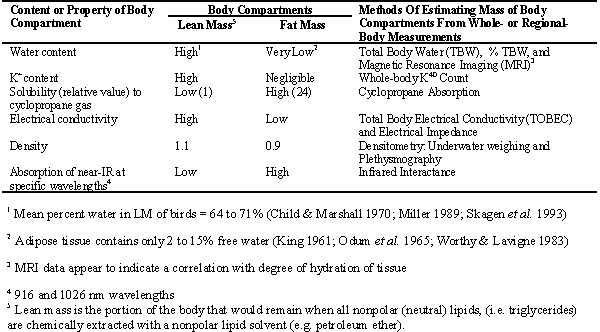
Most nonlethal methods are based upon a model that regards the body as two chemically distinct compartments: fat mass and lean mass. Lean mass is the sum of body water mass and fat-free dry mass, which is the portion of the body that would remain when all nonpolar (neutral) lipids, (i.e., triglycerides = fats) are chemically extracted with a nonpolar lipid solvent (e.g., petroleum ether). Some nonlethal methods estimate FM directly (referred to as the direct approach in this paper), whereas other methods estimate LM directly and then compute FM by difference (i.e., FM = BM - LM, where BM is body mass; referred to as the indirect approach in this paper). The properties and contents of adipose tissue differ from those of lean tissue in several ways, and most nonlethal methods of estimating FM or LM of animals recognize these differences (Table 1).
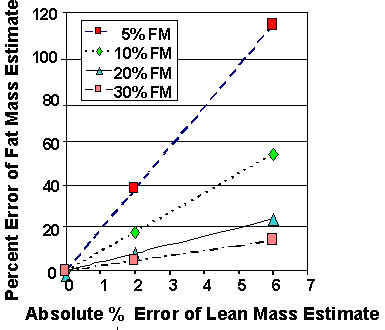
The following are examples of methods or measurements that have been used to estimate avian FM with the direct approach: fat scoring (Krementz & Pendleton 1990; Rogers 1991), near-infrared interactance of body surfaces and subcutaneous layers (Roby 1991), cyclopropane absorption (Gessaman et al.1998), whole-body nuclear magnetic resonance imaging (Mitchell et al. 1991), ultrasound (Baldassarre et al. 1980), percent total body water (Conway et al. 1994), and morphometrics (see Blem 1990). In contrast, nonlethal measurements that use the indirect approach to estimate FM include total body electrical conductivity (TOBEC, Roby 1991), whole-body K40 counts (Hinton et al. 1998), and total body water (TBW). In the indirect approach, small errors in LM can cause large errors in the estimate of FM because LM is usually much larger than FM. For example, a 2% error in the estimate of LM can produce a 38% error in the estimate of FM of a bird with only 5% body fat. The percent error decreases with increasing fatness of the bird (Fig. 1).
In this paper, I will review the rationale for using TBW (and %TBW), whole-body K40 counts, cyclopropane absorption, TOBEC, and morphometrics as estimators of LM and FM, along with findings of the most recent studies that have used these variables to predict avian body composition. Other estimators of avian body composition are discussed in the symposium paper that follows (Piersma & Klaassen 1999).
THE METHODS
Isotopic water dilution
Fats are stored in relatively nonhydrated adipose tissue containing only 2 to 15% free water (King 1961; Odum et al. 1965; Worthy & Lavigne 1983), and the percentage of water in the LM of birds has a narrow range (i.e., 64% to ~71%; Child & Marshall 1970; Robbins 1993:227; Skagen et al.1993). Thus, the percentage of water in a bird’s body (%TBW), which varies inversely with the percentage of fat mass (%FM, see Robbins 1993:fig. 11.2), can be used to predict %FM, then to compute FM (i.e., FM = %FM*BM/100). Percentage of body water also varies directly with %LM and, thus, could be used to predict LM and total body protein. Biologists have estimated FM from a measure of body water by both direct and indirect approaches.
In the direct approach, FM is computed from a regression equation relating %TBW to %FM, which has been determined from body composition analysis of a sampling of individuals from the study population. Several studies, particularly of waterfowl, have estimated FM of a large proportion of study birds from their %TBW (measured by drying the carcasses to constant weight), rather than by fat extraction, which is a more expensive and time consuming method of estimating FM (Bailey 1979; Wishart 1979; Johnson et al. 1985). This approach requires that a small proportion of the study birds of that species be fat-extracted to derive a relationship between %TBW and % FM, which can then be applied to other birds in the population.
In the indirect approach, TBW is measured (by either desiccation or isotope dilution) and LM is calculated by assuming that TBW is a fixed fraction (K) of LM (e.g., LM = TBW/K; mean K = 0.68 in 11 species of avian migrants, Child & Marshall 1970; mean K = 0.64 in 2 species of sandpipers, Skagen et al. 1993; mean K = 0.71 in Pintails Anas acuta, Miller 1989; mean K = 0.67 in 2 species of phalaropes, Ellis & Jehl 1991; and mean K = 0.70 in Sandhill Cranes Grys canadensis and 0.72 in Greater White-fronted Geese Anser albifrons, Johnson et al. 1985). The mean Ks for 12 species of waders were very similar; ranging from 0.67 to 0.70 (Piersma & Brederode 1990). Piersma (pers. comm.) has found that water content of lean body mass is always very close to 69% (68-70%), and that percentages lower than 68% are from dehydrated carcasses. He believes that the considerable variation in water content of lean mass reported among studies is due to methodological factors, especially the extent of dehydration of carcasses between sampling and analysis, the variation in gut content, and the possibility of water-inflated lungs in drowned carcasses. In contrast, mean K for mammals = 0.73 (Sheng & Huggins 1979). This approach has been used to estimate FM of several species of waterfowl including Redhead Ducks Aythya americana (Bailey 1979), American Wigeons Anas americana (Wishart 1979), Greater and Lesser Scaup Aythya marila and A. affinis (Chappell & Titman 1983), Northern Pintail Anas acuta (Miller 1989), Maned Duck Chenonetta jubata (Briggs & Thornton 1988), and the Wood Thrush Hylocichla mustelina (Conway et al. 1994). In all of these studies, TBW was measured by killing and drying the birds to constant mass. The error associated with FM of individual birds estimated by using a constant (K), however, could be significant (Sheng & Huggins 1979), since the fraction of water in LM among individuals within a species can cover a broad range (see Piersma & Brederode 1990).
Isotopes of water (2H2O, deuterium; 3H2O, tritiated water; or H218O) are often used to nonlethally estimate TBW by isotope dilution. The calculation of TBW volume is based upon the simple relationship C1V1 = C2V2, where C1V1 is the concentration and volume of isotope injected into or swallowed by the animal, C2 is the final concentration of isotope in blood at equilibrium and V2 is the TBW volume. The ratio of TBW measured by H218O, and by either 2H2O or 3H2O, to that measured by desiccation averages 1.01 and 1.04, respectively, for birds (Speakman 1997:table 7.4). The isotope dilution measurement of TBW has been used extensively to estimate body composition in humans (with an absolute accuracy of 1-2 %; Westerterp-Plantenga et al. 1994) and in other mammals (Sheng & Huggins 1979). TBW measured by isotopic water dilution has been used sparingly to estimate the FM of birds (poultry; Farrell & Balnave 1977; Johnson & Farrell 1988), and Cliff Swallows (Hirundo pvrrhonata; Gauthier & Thomas 1990). Gauthier & Thomas (1990) found that 3H2O dilution overestimated TBW measured by desiccation by 2.3±0.3%, consistent with previous findings on birds (Degan et al. 1981; Crum et al. 1985), and also that TBW was sufficiently precise to estimate and compare the mean fat reserves between groups of birds , although the errors of estimating FM in individuals ranged from 25 to 150%. Though TBW is commonly measured by isotopic water dilution in studies of free-living birds, in conjunction with measurement of CO2 production and water flux using the doubly-labeled water (DLW) method (Speakman 1997), this approach has not been used to estimate avian FM in studies using DLW.
Groscolas et al. (1991) demonstrated that BM and TBW (by isotope dilution), measured periodically during the 17-day fast of an incubating Great-winged Petrel (Pterodroma macroptera), provides a good estimate of protein and fat utilization. They then computed daily energy expenditure from the mass losses of both fat and protein and reasonable estimates of their energy densities. Four assumptions underlie this method: (1) that neither water nor protein is lost from plumage and bones; (2) that loss of all other components from LM, such as carbohydrates, structural lipids, and minerals is negligible; (3) that water and protein are lost in direct proportion to their content in LM; and (4) that the loss of LM is only from water and protein. In short, LM and protein loss are computed from TBW loss, and FM loss is computed indirectly from LM and BM loss.
The empirical relationship between percentage of total body protein and %TBW measured in 8 dead Gray Seals Halichoerus grypus is now being used to estimate total body protein of living gray seals, whose TBW is measured by isotopic water dilution (Reilly & Fedak 1990). The accuracy and potential usefulness of this empirical approach, which has not been used to estimate LM or total body protein of live birds, needs to be evaluated on birds using two groups of individuals - a derivation group and a test group.
The TBW method of estimating LM and FM has several limitations. First, it is technically invasive, rather than noninvasive, since the bird is injected with an isotope of water and a blood sample taken about 2 hr afterwards when the isotope has reached an equilibrium with all body water compartments. When using H218O or 2H2O it is desirable to also take a blood sample before the injection of the isotope so that the background level of the isotope will be known. Second, estimation of the FM of an individual of a single species from a relationship between %FM or %LM and %TBW requires killing 20 to 40 birds in the population to obtain data for deriving the relationship. Studies of the body composition of marine mammals using TBW measured by isotopic water dilution show that these empirical relationships differ between pups and adults of the same species (Arnould et al. 1996) and among species (Reilly & Fedak 1990); use of species-specific relationships provide the best accuracy. Third, the accuracy of estimating body composition is affected by the dehydration level of the bird. Further research is needed to assess the accuracy of using body water, measured by isotope dilution, to nonlethally estimate avian LM, FM, and total body protein, perhaps in conjunction with morphometric variables in multiple regression relationships.
Whole-body potassium-40
Potassium occurs in lean tissue but not in fat (Coward et al. 1988). Whole-body counting of potassium-40, which emits characteristic gamma radiation at 1.46 MeV and comprises a fixed percentage of total potassium in the body of animals (e.g., 0.012% in humans; Westerterp-Plantenga et al. 1994), has been used to estimate FM of animals by the indirect method. Lean mass of humans has been estimated from 40K analyses within 3% accuracy (Westerterp-Plantenga et al. 1994). Lean mass and FM of several species of large domestic mammals (pigs, Schmidt et al. 1974; Domermuth et al. 1976: steers, Clark et al. 1976: dairy cows, Belyea et al. 1978) have been computed using empirically-derived relationships between LM (or FM) as the dependent variable, and two or more independent variables, which include 40K counts and body mass. Only three studies have evaluated the accuracy of estimating FM (or LM) of small animals from their whole body 40K count, two of these on birds. Whole-body assay of 40K was not successful in accurately predicting FM of 1 to 77 day-old Wood Ducks (Aix sponsa; Clay et al. 1979). In a study of 39 Rock Doves Columba livia, whole-body counts of 40K, when used alone, were not useful predictors of LM or FM (Hinton et al. 1998). The small body size of Rock Doves, and thus their low level of 40K, seems to be the major cause of the low correlations between 40K counts and LM. Since the lean tissue of the Rock Doves contained 2.6 mg of K g-1 (about the same as found in the lean mass of humans, 2.5 and 2.3 mg of K g-1 lean mass for man and women, respectively; Behnke & Wilmore 1974), a 500-g Rock Dove with a body fat of 10% would have only about 1 g of total body potassium (TBK). Potassium-40 whole-body counts have been relatively successful in predicting LM, FM, and TBK of mammals much larger than Rock Doves; however, researchers have had limited success with this method on animals weighing <5 kg (i.e., <10 g of TBK). Schmidt et al. (1974) found that piglets weighing 1.2 kg were too small to obtain adequate 40K counts with the limitations of their equipment; the smallest pigs they counted successfully weighed 5.4 kg.
Current whole-body counting technology might be successfully used on large birds (e.g., penguins), whose 40K whole-body count should be appreciably above levels inherent in the background of the detector system. The disadvantages of this method are that it (1) requires expensive equipment (the chamber together with the scintillation counter may cost >$40,000); (2) requires heavy equipment (a whole-body counting chamber lined with lead weighing several hundred pounds, which is thus not portable); (3) requires counting times of several hours; (4) requires calibration by killing and fat extracting birds, and (5) explains only a small percentage of the variation in FM not explained by morphological measurements. Therefore, the difficulties of using this method in the field on live birds, along with its weak power for predicting either LM or FM, outweigh the advantage of being totally noninvasive.
Total body electrical conductivity
The total body electrical conductivity (TOBEC) index--a measurement of electrical conductive properties of the body--is correlated with LM and TBW since the conductive property of LM is relatively large compared to that of FM. In 1988 Walsberg showed a high correlation between the TOBEC index and LM in birds ranging in BM from 14 to 170 g. Although he did not evaluate the accuracy of the TOBEC index for estimating FM by the indirect approach, he suggested it ‘may be quite useful for biologists interested in quantifying lipid stores.’ Since 1988 several studies have evaluated this method on birds up to about 275 g by using three different models of TOBEC instruments (the EM-SCAN SA-l, SA-2, and SA-3000 series of Small Animal Body Composition Analyzers; Castro et al. 1990; Roby 1991; Morton et al. l991; Scott et al. 1991; Skagen et al. 1993; Conway et al. 1994; Meijer et al. 1994; Asch & Roby 1995; Lyons & Haig 1995; Burger 1997). Most of these studies have shown that the error of using a TOBEC index alone as a predictor of LM and FM can be large (Table 2). These evaluations have found that TOBEC indices explain a much smaller fraction of the variation in avian FM than body morphometrics; however, the TOBEC index used with morphometrics in a FM predictive model may marginally improve the accuracy of the model (Skagen et al. 1993; Lyon & Haig 1995; Burger 1997).
Other limitations of the TOBEC method include the following. First, the TOBEC index can be affected by factors other than the LM of the bird, e.g., body temperature (Scott et al. 1991; Harden 1993), dehydration (Walsberg 1988; Roby 1991), position of bird in the measurement chamber (Scott et al. 1991), and motion of bird during the index measurement; in addition, the index was affected in two of four studies by the metal identification band on the bird (Scott et al. 1991). Therefore, these variables must be held constant during the instrument calibration procedure and during subsequent TOBEC measurements; otherwise, the accuracy of this method is compromised. Second, equations relating TOBEC indices and LM that are derived from data on several avian species (see Walsberg 1988; Castro et al. 1990; Scott et al. 1991) are not very accurate for single species studies (Scott et al. 1991; Skagen et al. 1993; Lyon & Haig 1995). Therefore, 20 to 40 individuals of a single species must be killed and fat extracted after measuring their TOBEC index to obtain calibration data, which can then be used to nonlethally estimate LM and FM of others in the population. Third, a quadratic equation best describes the relationship between LM and the TOBEC index measured with a SA-1 or SA-2 analyzer, causing the precision of the prediction equation to be lower for smaller species because TOBEC values correspond to a wider range of LM (Asch & Roby 1995). A small change in LM is easily detectable in a bird >200 g, but not in a bird <100 g; therefore, doubling of FM in species the size of House Sparrows Passer domesticus, (mean BM = 29 g; mean %FM = 4.0) or European Starlings Sturnus vulgaris (mean BM = 89 g; mean %FM = 4.0) may not be detectable by the SA-1 or SA-2 analyzers, due to the error in estimating LM from TOBEC index (Asch & Roby 1995). Finally, calibrations reported for one model of instrument (e.g, EMSCAN SA-2) will not be valid for a later model (i.e., the EM-SCAN SA-3000 series; Gessaman unpublished).
The advantages of the TOBEC method are that (1) it is totally noninvasive, (2) a series of five measurements can be made on one bird within 3 to 5 min, and (3) the instrument is ready to use when received from the manufacturer, easy to use and transport, and can be powered by a 12-volt car battery.
Cyclopropane absorption
Cyclopropane gas is about 24 times more soluble in fat than in lean tissue (Lesser et al. 1952, Blumberg et al. 1952). The concentration of cyclopropane gas in an airtight chamber containing an animal, 1.5% cyclopropane, and 98.5% oxygen decreases as the animals breathes in the cyclopropane, which dissolves into the FM and LM of the animal. The animal's FM is then calculated from measures of the initial and final equilibrium concentrations of cyclopropane in the animal chamber. The cyclopropane absorption method has been validated on only three species of small animals: laboratory rats Rattus norvegicus, pond turtles Trachemys scripta, and Rock Doves Columba livia. The error in the estimate of the FM in these three studies was 5.6% (mean FM = 13.2% of body mass [BM], range 8.1 to 18.9%) for 10 laboratory rats (Lesser et al. 1952); only 10%, despite low FMs (0.7 to 3.5% of BM) for 6 pond turtles (Henen 1991); and 11% (FM ranged from 5 to 22% of BM) for 40 Rock Doves (Gessaman et al. 1998). Although a mean error of 11% in estimating avian body fat is impressively small when compared with the error reported by some users of the TOBEC method, I recommend that potential users of this method carefully consider the limitations of an 11% error in estimating body fat before they attempt to use this moderately difficult method. Gessaman et al. (1998) list a few advantages and many disadvantages of this user-unfriendly method and provide detailed recommendations for future users. The method is probably not suitable for endotherms weighing <20 g where oxygen would be consumed rapidly from the chamber and would require frequent injections of oxygen into the chamber. This method does not require a calibration based on fat extracted from dead birds. The accuracy and precision of a system assembled to measure the fat mass of live birds can be evaluated with olive oil standards. The accuracy of estimating fat mass of a living bird by cyclopropane absorption seems to be dictated, in large part, by the analytical equipment and procedures used rather than by the bird. The error of this method for estimating avian FM could probably be reduced to 5 to 10% by using optimal analytical equipment and procedures.
Morphometrics
Brown (1996) presents the rationale for using body mass, body mass corrected for structural size, and structural measurements alone as independent variables in regression models for predicting LM, FM, and nutrient stores, reserves, and non-reserve structural parts of birds. Many researchers have estimated FM by either the direct or indirect approach using morphometrics alone (e.g., body mass, and wing, head, tarsus or body length), or in conjunction with other measurements (e.g., TOBEC, Skagen et al. 1993; abdominal fat, Bailey 1979, Piersma 1984; see Blem 1990 for a review). The regression equations in both approaches are derived empirically from FM and LM measured by fat extraction and have been shown to differ among species and within species by sex, geographical origin of individuals, and time of year. Morphometrics explain the majority of the variance in avian FM (Skagen 1993; Burger 1997; Hinton et al. 1998), but can also be coupled with other independent variables in a model to improve the accuracy of the estimate of FM. For example, Skagen et al. (1993) reported that body mass, wing length, and head length explained about 90% of the variance in fat mass of Semipalmated Sandpipers Calidris pusilla and White-rumped Sandpipers C. fuscicollis, and TOBEC indices explained an additional 7.8 and 2.6% for these two species, respectively. In another example, the error of estimating LM and FM of Rock Doves from their morphometrics alone was 3% and 18%, respectively (Hinton et al. 1998) but was reduced to 2% and 12%, respectively, when TOBEC and body water were included as independent variables with body mass, wing length, and body length (Gessaman unpublished). The lowest percentage of error reported for estimating LM and FM from morphometrics alone is about 3% and 15%, respectively (Table 3). Burger (1997) showed that the absolute error of estimating FM from predictive models, by either the direct or indirect method, was the same when using the same multiple independent variables, as long as they included body mass, that is, it is not necessary to evaluate the accuracy of both the indirect and direct approach of estimating FM when body mass is one of the independent variables in both predictive models.
Van der Meer & Piersma (1994) argued that the sources of error in estimating or predicting the fat or lean composition of birds are not the same for all species and, therefore, that a single or general approach as typically used and as referenced above (e.g., fat or lean mass = f1 (body mass) + f2 (body size) + f3 (TOBEC)) is inappropriate because of the ‘arguable ways in which the error structures of the predictive models are handled.’ In a detailed theoretical and empirical analysis of this problem, they partitioned body composition into two compartments: body stores and body structural parts. Stores are the nutrients accumulated in anticipation of periods of shortage, some birds storing both fat and protein. The structural part is composed of non-reserves and reserves, which are nutrients used only in emergencies because they are structural parts necessary for a functionally ‘normal’ life (Lindström & Piersma 1993). The reserves are mostly structural protein, but probably include structural lipids. Van der Meer & Piersma (1994) showed that a breakpoint model (at breakpoint, the body mass of a starving bird equals the mass of the structural part), which assumes all variation is due to unexplained differences in the structural mass, is most appropriate when the variation in the structural mass is large. In contrast, when only variation in the composition of the stores is large, as seems to be true for the Greater Golden Plover Pluvialis apricaria, a second model, which assumes that all variation is due to unexplained differences in the composition of the stores, is more appropriate. Future studies of sources of variation in the size and composition of the structural body and of nutrient stores are required to verify the assumptions of these models and to evaluate the possible improvements of this approach over the typically used predictive (regression) models for estimating nutrient content. It is not clear whether viewing the body as having three compartments (stores, reserves, and non-reserve structural parts) or four compartments (fat stores, structural fat, protein stores, and structural protein), or more than four compartments, rather than two compartments (i.e., FM and LM) would improve the accuracy of estimating energy and nutrient flux.
Although many earlier studies on avian body composition used only coefficients of determination (r2) to evaluate the relative usefulness of equations that predicted either FM or LM from independent variables, this practice should be discouraged in future research. Regression equations cannot be evaluated for their usefulness simply by comparing their coefficients of determination. For example, absolute errors in predicting lean and fat masses for interspecific equations with r2 > 0.95 were greater than for single-species equations with r2 < 0.80 (Skagen et al. 1993). All future evaluations of nonlethal methods, which involved predictive equations, should include two groups of birds (a derivative group and a test group). Predictive equations are computed from data on derivative birds then percent error of these equations is evaluated from data on the test group (see Roby 1991, Skagen et al. 1993, Lyon & Haig 1995; Burger 1997).
All of the errors shown for the different methods in this paper are mean errors. The error of predicting FM or LM of a single individual with any of these methods should be larger, since accuracy of a prediction of the mean for an entire population is greater than that for an individual (Zar 1984).
SUMMARY
(1) Nearly all of the nonlethal methods of estimating LM or FM described in this paper require calibration equations derived from data on dead birds. These calibration equations have been shown to differ among species and, in some cases, within a species by sex, geographical origin of individuals and time of year. The exceptions are the cyclopropane method, which can be calibrated with olive oil rather than bird fat, and the isotopic water dilution method of measuring TBW, then computing LM, which assumes the ratio of TBW/LM is a known constant among birds.
(2) Generally, the percentage of error in estimating FM by the indirect approach (i.e., estimating LM, then computing FM from FM = BM – LM) is much larger than by the direct approach
.(3) The isotopic water dilution method of estimating the LM of a live bird from TBW and its %FM from %TBW has been used in only a few avian studies. Previous studies of the relationship between these variables in dead birds shows that body water can explain some of the variability in LM or FM not explained by easily measured morphometric variables. Percentage of LM and total body protein have been estimated with the isotopic water dilution method from %TBW in marine mammals, but not in birds. The accuracy of this nonlethal approach for estimating LM, FM, and total body protein deserves evaluation on a broad range of avian species.
(4) Whole-body potassium-40, which is radioactive and occurs naturally as a fixed fraction of whole body potassium, is used to estimate body composition of humans with only 1-2% error. This method is not applicable for most birds because their body potassium-40 content is too low.
(5) The error of predicting LM from the TOBEC index alone ranges from 1 to 9 %, whereas the error of predicting FM from the TOBEC index alone ranges from 10% to >300%. In some studies, the TOBEC index has explained 2 to 8% of the variance in FM not accounted for by body mass or body size parameters in multiple regression equations.
(6) The cyclopropane absorption method, which measures fat mass with a mean error ranging from 5.6% in laboratory rats to 11% in Rock Doves seems to be more accurate than either the TOBEC, morphometric or isotopic water dilution methods; however, it is neither user friendly nor easy to use in the field.
(7) The multiple regression approach to estimate LM or FM using combinations of independent variables such as body mass, body size parameters, TOBEC indices, and body water, generally explains more than 90% (and up to 98%) of the variance in LM and FM. Although this general approach lacks a theoretical basis and has been labeled an inappropriate approach (Van de Meer & Piersma 1994), this method must be currently regarded as the most accurate nonlethal approach easily used in the field. This generalized approach does not, however, incorporate our current understanding of the dynamics of differential protein and fat metabolism during long-term fasting, which includes the breakpoint concept. Perhaps viewing the body composition as having more than two compartments (i.e., LM and FM) would improve the accuracy of estimating energy and nutrient flux.
ACKNOWLEDGEMENTS
I want to thank Theunis Piersma for comments on a draft of this paper.
REFERENCES
Arnould, J.P.Y., Boyd, I.L. & Speakman, J.R. 1996. Measuring the body composition of Antarctic Fur Seals (Arctocephalus gazella): validation of hydrogen isotope dilution. Physiological Zoology 69:93-116.
Asch, A. & Roby, D.D. 1995. Some factors affecting precision of the total body electrical conductivity technique for measuring body composition in live birds. Wilson Bulletin 107:306-316.
Bailey, R.O. 1979. Methods of estimating total lipid content in the Redhead Duck (Aythya americana) and an evaluation of condition indices. Canadian Journal of Zoology 57:1830-1833.
Baldassarre, G.A., Whyte, R.J. & Bolen, E.G. 1980. Use of ultrasonic sound to estimate body fat depots in the mallard. Prairie Naturalists 12:79-86.
Behnke, A.R. & Wilmore, J.H. 1974. Evaluation and regulation of body build and composition. Englewood Cliffs, New Jersey; Prentice-Hall.
Belyea, R.L., Frost, G.R., Martz, F.A. and Forkner, L.G. 1978. Body composition of dairy cattle by potassium-40 liquid scintillation detection. Journal of Dairy Science 61:206.
Blem, C.R. 1990. Avian energy storage. In: Power, D.M. (ed), Current Ornithology. Vol. 7; New York; Plenum Press:59-113.
Blumberg, A.G., La Du, Jr., B.N., Lesser, G.T. & Steele, J.M. 1952. The determination of the solubility of cyclopropane in fats and oils with the use of the warburg apparatus. Journal of Pharmacology and Experimental Therapeutics 104:325-328.
Briggs, S.V. & Thornton, S.A. 1988. Abdominal fat and percentage water as predictors of body fat in adult maned duck, Chenonetta jubata. Australian Wildlife Research 15:231-234.
Brown, W.D. 1996. Assessing body condition in birds. In: Nolan, V., Jr. & Ketterson, E. (eds.) Current Ornithology, Vol. 13; New York; Plenum Press: 67-135.
Burger, M.E. 1997. Estimating lipid and lean masses in a wintering passerine: an evaluation of TOBEC. The Auk 114:762-769.
Castro, G., Wunder, B.A. & Knopf, F.L. 1990. Total body electrical conductivity (TOBEC) to estimate total body fat of free-living birds. The Condor 92:496-499.
Chappell, W.A. & Titman, R.D. 1983. Estimating reserve lipids in Greater Scaup (Aythya marila) and Lesser Scaup (A. affinis). Canadian Journal of Zoology 61:35-38.
Cherel, Y. & Groscolas, R. 1999. Relationships between nutrient storage and nutrient utilization in long-term fasting birds and mammals. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban:17-34. Johannesburg: BirdLife South Africa.
Child, G.I. & Marshall, S.G. 1970. A method of estimating carcass fat and fat-free weights in migrant birds from water content of specimens. The Condor 72:116-119.
Clark, J.L., Hedrick, H.B. & Thompson, G.B. 1976. Determination of body composition of steers by potassium-40. Journal of Animal Science 42:352-356.
Clay, D.L., Brisbin, Jr., I.L. & Youngstrom, K.A. 1979. Age-specific changes in major body components and caloric values of growing Wood Ducks. The Auk 96:296-305.
Conway, C.J., Eddleman, W.R. & Simpson, K.L. 1994. Evaluation of lipid indices of the Wood Thrush. The Condor 96:783-790.
Coward, W. A., Parkinson, S.A. & Murgatroyd, P.R. 1988. Body composition measurements for nutrition research. Nutrition Research Reviews 1:115-124.
Crum, B.G., Williams, J.B. & Nagy. K.A. 1985. Can tritiated water-dilution space accurately predict total body water in Chukar Partridges? Journal of Applied Physiology 59:1383-1388.
Degen, A.A., Pinshow, B., Alkon, P.U. & Arnon, H. 1981. Tritiated water for estimating total body water and water turnover rate in birds. Journal of Applied Physiology 51:1183-1188.
Domermuth, W., Veum, T.L., Alexander, M.A., Hedrick, H.B., Clark, J. & Eklund, D. 1976. Prediction of lean body composition of live market weight swine by indirect methods. Journal of Animal Science 43:966-976.
Ellis, H.I. & Jehl, J.R.,Jr. 1991. Total body water and body composition in phalaropes and other birds. Physiological Zoology 64:973-984.
Farrell, D.J. & Balnave, D. 1977. The in vivo estimation of body fat content in laying hens. British Poultry Science 18:381-384.
Gauthier, M. & Thomas, D.W. 1990. Evaluation of the accuracy of 22Na and tritiated water for the estimation of food consumption and fat reserves in passerine birds. Canadian Journal of Zoology 68:1590-1594.
Gessaman, J.A., Nagle, R.D. & Congdon, J.D. 1998. Evaluation of the cyclopropane absorption method of measuring avian body fat. The Auk 115:175-187.
Groscolas, R., Schreiber, L. & Morin, F. 1991. The use of tritiated water to determine protein and lipid utilization in fasting birds: a validation study in incubating Great-winged Petrels, Pterodroma macroptera. Physiological Zoology 64:1217-1233.
Harden, S.M. 1993. Fat content of American Kestrel (Falco sparverius) and sharp-shinned hawks (Accipiter striatus) estimated by total electrical conductivity. MS Thesis, Utah State University, Logan, Utah.
Henen, B.T. 1991. Measuring the lipid content of live animals using cyclopropane gas. American Journal of Physiology 261:R752-R759.
Hinton, T.G., Gessaman, J.A., Nagle, R.D. & Congdon, J.D. 1998. An evaluation of whole body potassium-40 content for estimating lean and fat mass in pigeons. The Condor 100: 579-582.
Johnson, D.H., Krapu, G.L., Reinecke, K.J. & Jorde, D.G. 1985. An evaluation of condition indices for birds. Journal of Wildlife Management 49:569-575.
Johnson, R.J. & Farrell, D.J. 1988. The prediction of body composition in poultry by estimation in vivo of total body water with tritiated water and deuterium oxide. British Journal of Nutrition 59:109-124.
King, J.R. 1961. The bioenergetics of vernal premigratory fat deposition in the white-crowned sparrow. The Condor 63:128-142.
Krementz, D.G. & Pendleton, G.W. 1990. Fat scoring: sources of variability. The Condor 92:500-507.
Lesser, G.T., Blumberg, A.G. & Steele, J.M. 1952. Measurement of total body fat in living rats by absorption of cyclopropane. American Journal of Physiology 169:545-553.
Lindström, A. & Piersma, T. 1993. Mass changes in migrating birds: the evidence for fat and protein storage re-examined. Ibis 135:70-78.
Lyons, J.E. & Haig, S.M. 1995. Estimation of lean and lipid mass in shorebirds using total-body electrical conductivity. The Auk 112:590-602.
Meijer, T., Möhring, F.J. & Trillmich, F. 1994. Annual and daily variation in body mass and fat of Starlings Sturnus vulgaris. Journal of Avian Biology 25:98-104.
Miller, M.R. 1989. Estimating carcass fat and protein in Northern Pintails during the nonbreeding season. Journal of Wildlife Management 53:123-129.
Mitchell, A.D., Wang, P.C., Rosebrough, R.W., Elsasser, T.H. & Schmidt, W.F. 1991. Assessment of body composition of poultry by nuclear magnetic resonance imaging and spectroscopy. Poultry Science 70:2494-2500.
Morton, J.M., Kirkpatrick, R.L. & Smith, E.P. 1991. Comments on estimating total body lipids from measures of lean mass. The Condor 93:463-465.
Odum, E.P., Marshall, S.G. & Marples, T.G. 1965. The caloric content of migrating birds. Ecology 46:901-904.
Piersma, T. 1984. Estimating energy reserves of Great Crested Grebes Podiceps cristatus on the basis of boy dimensions. Ardea 72:119-126.
Piersma, T. & van Brederode, N.E. 1990. The estimation of fat reserves in coastal waders before their departure from northwest Africa in spring. Ardea 78:221-236.
Piersma, T. & Klaassen, M. 1999. Methods of studying the functional ecology of protein and organ dynamics in birds. In: Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban: 36-51. Johannesburg: BirdLife South Africa.
Pond, C.M. 1981. Storage. In: Townsend, C.R. & Calow, P.(eds) Physiological ecology: an evolutionary approach to resource use. Sunderland, Massachusetts; Sinauer: 190-219.
Reilly, J.J. & Fedak, M.A. 1990. Measurement of the body composition of living gray seals by hydrogen isotope dilution. Journal of Applied Physiology 69:885-891.
Robbins, C.T. 1993. Wildlife feeding and nutrition. 2nd ed; San Diego; Academic Press: 352 pp.
Roby, D.D. 1991. A comparison of two noninvasive techniques to measure total body lipid in live birds. The Auk 108:509-518.
Rogers C.M. 1991. An evaluation of the method of estimating body fat in birds by quantifying visible subcutaneous fat. Journal of Field Ornithology 62:349.
Schmidt, M.K., Clark, J.L., Veum, T.L. & Krause, G.F. 1974. Prediction of composition of crossbred swine from birth to 136 kg live weight via the liquid scintillation whole body counter. Journal of Animal Science 39:855-864.
Scott, I., Grant, M. & Evans, P.R. 1991. Estimation of fat-free mass of live birds: use of total body electrical conductivity (TOBEC) measurements in studies of single species in the field. Functional Ecology 5:314-320.
Sheng, H. & Huggins, R.A. 1979. A review of body composition studies with emphasis on total body water and fat. American Journal of Clinical Nutrition 32:630-547.
Skagen, S.K., Knopf, F.L. & Cade, B.S. 1993. Estimation of lipids and lean mass of migrating sandpipers. The Condor 95:944-956.
Speakman, J.R. 1997. Doubly labelled water: theory and practice. London; Chapman & Hall: 399 pp.
Van der Meer, J. & Piersma, T. 1994. Physiologically inspired regression models for estimating and predicting nutrient stores and their composition in birds. Physiological Zoology 67:305-329.
Walsberg, G.E. 1988. Evaluation of a nondestructive method for determining fat stores in small birds and mammals. Physiological Zoology 61:153-159.
Westerterp-Plantenga, M.S., Fredrix, E. & Steffens A.B. 1994. Food intake and energy expenditure. Boca Raton, Florida; CRC Press: 408pp.
Wishart, R.A. 1979. Indices of structural size and condition of American Wigeon (Anas americana). Canadian Journal of Zoology 57:2369-2374.
Worthy, G.A.J. & Lavigne, D.M. 1983. Energetics of fasting and subsequent growth in weaned harp seal pups, Phoca groenlandica. Canadian Journal of Zoology 61:447-456.
Zar, J.H. 1984. Biostatistical analysis. 2nd ed; Englewood Cliffs, New Jersey; Prentice-Hall:620 pp.
Table 1. Most non-lethal methods of estimating body fat of animals are based upon a model that regards the body as two chemically distinct compartments -- fat mass (FM) and lean mass (LM). Some non-lethal methods estimate FM directly, while other methods estimate LM directly and then compute FM by difference (i.e., FM = BM - LM, where BM is body mass). This table shows several ways in which the contents or properties of fat tissue differ from those of lean tissue; most of the methods for estimating FM or LM of animals capitalize on one or more of these differences.

Table 2. These are results from three studies that used TOBEC indices alone to estimate lean mass and then to compute fat mass by the indirect method. They clearly illustrate that the error of estimating fat mass by the indirect method is much larger than the error of estimating lean mass. These results follow the trends shown in Figure 1.
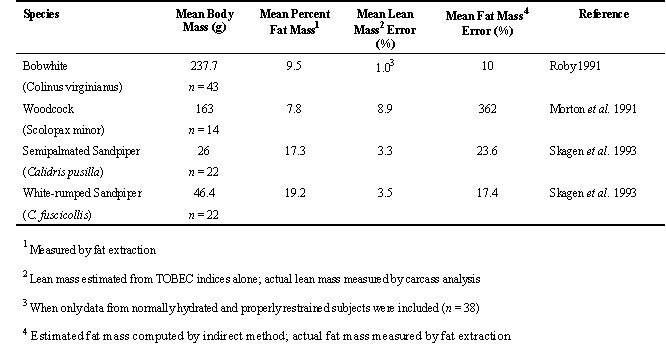
Table 3. A comparison of the error of predicting FM (fat mass) from regression equations containing only morphometric independent variables (the lowest error is about 15%) with that from regression equations containing independent variables that include TOBEC indices and morphometric parameters (the lowest error is about 13%).
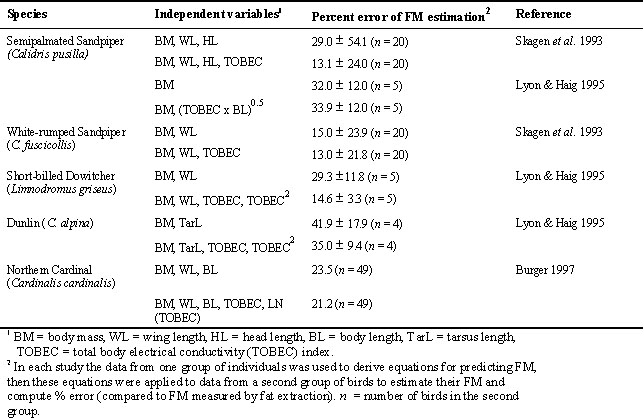
Fig. 1. When using the indirect method to estimate fat mass (FM) from an estimate of lean mass (LM, e.g., FM = BM-LM), the percent of the FM estimate will nearly always be greater than the percent error of the LM estimate because LM is nearly always more than 50% of BM (the absolute error of estimating LM and FM by the indirect method is the same). The relative error of the FM estimate is a function of the absolute error of the LM estimate and the percent FM (100*FM BM-1) of the bird. This figure illustrates that even a small percent error in the estimate of the lean mass, e.g., 2%, produces a large percent error (38%) in the estimate of the fat mass of a bird with 5% fat mass. The percent error of the estimate of FM decreases to 18, 8, and 4.7% with increasing fatness to 10, 20, and 30%, respectively. The relative error of the estimate of FM is shown for percent errors in estimating LM of up to 6%.